Abstract
Introduction. Tyrosine kinase inhibitors (TKIs) have revolutionized chronic phase (CP) chronic myeloid leukemia (CML) care with many patients achieving major and deeper molecular responses. However, for those who are resistant to or do not tolerate the approved TKIs, there are few alternatives. We therefore developed a custom high throughput drug screen comprised of both FDA approved and investigational agents.
Methods. Fifty-six samples (50 individual patients) have undergone testing in the drug sensitivity assay, for which a large fraction exhibited resistance to approved agents. The Quellos High Throughput Core Laboratory's Cancer Drug Sensitivity has been CLIA approved for leukemia since 2015. Blood and bone marrow samples were obtained from CML patients with written informed consent. Mononuclear cells were isolated by density depletion. The myeloid population was obtained by lineage depletion of non-myeloid cells using magnetic beads and antibodies to erythroid lineage (CD235a), T (CD3) and B (CD19) lymphocytes, and NK (CD56) cells. Flow cytometry confirmed successful enrichment of the myeloid cell population. Cells were plated on extracellular matrix coated 384 well plates to test under conditions of adhesion mediated chemotherapy resistance. Initially, the assay was comprised of 32 drugs (11 patients) selected based on published activity in CML and resistant CML. The assay was then expanded to 64 drugs. Compounds are added (ranging from 5 pM to 100 μM) to patient samples using the CyBio CyBi-Well Vario and incubated at 37°C, 5% CO2 for 72 hours, then viability is assessed by CellTiterGlo. IC50s and AUCs are calculated for each drug using XLFit (IDBS) and a standard 4 parameter logistical model. Transcriptome analysis is planned for these samples.
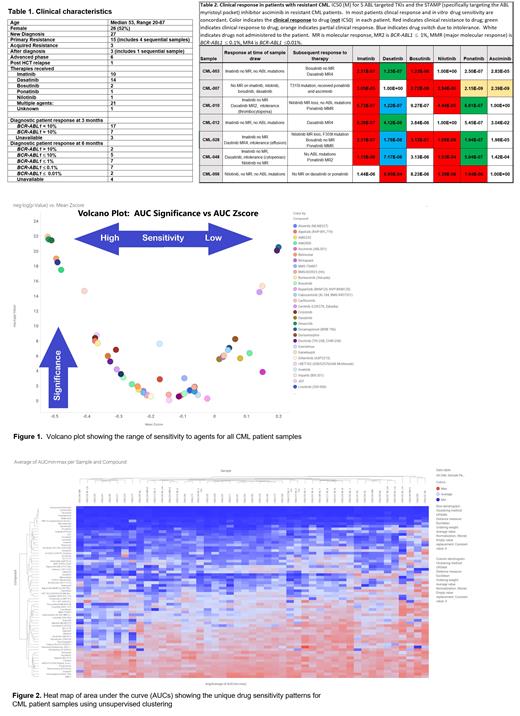
Results. Clinical characteristics are shown in Table 1. Mean and median BCR-ABL1 transcripts were 69% and 70% in diagnosis samples and 63% and 55% in resistant samples, respectively (P=0.607). ABL mutations were present in 5 patients (M244V, T315I, F359I). Additional myeloid mutations were present in 5 of 6 evaluable advanced phase samples, 4 of 17 evaluable diagnostic samples, and 3 of 10 evaluable resistant samples and included ASXL1, DNMT3A, IDH1, JAK2V617F, NRAS, RUNX1, and TET2. Figure 1 illustrates the breadth of sensitivity to agents in the assay. Figure 2 is a heat map of area under the curve (AUCs) illustrating the unique drug sensitivity patterns for all patients, with unsupervised clustering.
For new diagnosis patients, the TKIs imatinib, dasatinib, nilotinib, bosutinib, and ponatinib ranked in the top 8 drugs. For primary resistant patients, the IC50 values for imatinib, nilotinib, bosutinib, and ponatinib were higher than the new diagnosis patients. For example, for ponatinib, the mean IC50 was 402.6 ± 354.7 X 10 E-9 M for primary resistant samples vs. 1.65 ± 0.45 X 10 E-9 M for diagnosis group, p=0.015 (Welch t test), or about 250-fold higher (less sensitive). In accelerated and blast phase samples drugs with IC50 values lower than 0.1 µM, a range that could correlate with in vivo drug response, were identified for all patients (range, 3-20 drugs per patient). Top candidates included proteasome and kinase inhibitors. In 2 patients harboring NRAS mutations, IC50 for trametinib was less than 0.1 µM as compared to patients without NRAS mutations, where the IC50s were higher. Clinical outcomes are available for nearly all patients. Although the study was not designed to select next-line TKI therapy in resistant patients, drug profiling was informative in many cases. Data for 7 resistant patients are shown in Table 2. For example, CML-012 had the lowest IC50 value (indicating most sensitive) for dasatinib, 4.1 X 10E-8 M and responded to dasatinib after failing imatinib (IC50 8.4 X 10E-7M). CML-003 did not respond to bosutinib (IC50 1.2 X 10E-6M) and did respond to dasatinib (IC50 1.2 X 10E-7M). CML-056 did not respond to nilotinib (IC50 1.4 X 10E-6M), dasatinib (IC50 6.9 X 10E-4M), or ponatinib (IC50 1.0 X 10E-6M). Notably, in all resistant patient samples we identified drugs with IC50 values lower than 0.1 µM. These therapeutics can be prioritized for further evaluation, either alone or in combination with TKIs, in resistant CML patients.
Conclusion. In vitro drug sensitivity testing provides data for potential agents for patients with resistance or intolerance to FDA approved TKIs, or those that have entered accelerated phase or blast phase.
Oehler: Blueprint Medicines: Consultancy; Takeda: Consultancy; Pfizer: Research Funding; OncLive: Honoraria; BMS: Consultancy. Becker: Abbie: Research Funding; SecuraBio: Research Funding; Cardiff Oncology: Research Funding; Pfizer: Research Funding; BMS: Research Funding; CVS Caremark: Consultancy; Glycomimetics: Research Funding.
We developed a custom high throughput drug screen comprised of both FDA approved and investigational agents